Research Snippet #8: Testicular Cancers
All of the information in this research snippet has been obtained from a review by Leãoet al “Testicular cancer Biomarkers: A role of Precision Medicine in Testicular cancer”.
A link can be found here: https://pubmed.ncbi.nlm.nih.gov/30497810/
This is only a brief overview and would highly recommend reading the article and attending our CSM Testicular Cancer webinar to get the most out of your learning 😄
First let us revise the main histologic types of testicular cancer!
Testicular germ cell tumours (TGCTs) account for 90-95% of all testicular tumours, and are subdivided into seminoma tumours (60% of TGCTs) and non-seminomatous germ cell tumours (NSGCTs, 40% of TGCTs). TGCTs are most common in young men aged 15 -34 years. The remaining non-germ cell tumours account for 5-10% of testicular tumours and include sex cord & gonadal stromal tumours (i.e. Leydig cell tumours, Sertoli cell tumours), lymphoid & hematopoietic tumours, and metastatic tumours.
NSGCTs are divided into embryonal carcinomas, yolk sac carcinomas, choriocarcinomas and teratomas which are traditionally associated with specific biomarkers. These include: AFP for Yolk sac carcinomas, AFP+ b-HCG for embryonal carcinomas, and no markers for teratomas. However, the majority of TGCTs display heterogeneity in histology, often containing more than 1 histology, and are labelled as mixed germ cell tumours. Furthermore, these current tumour markers have low sensitivity and accuracy values as both predictive and diagnostic markers.
A summary table of main histologic types of testicular cancers is displayed below in Table 1 obtained from Leão et al.
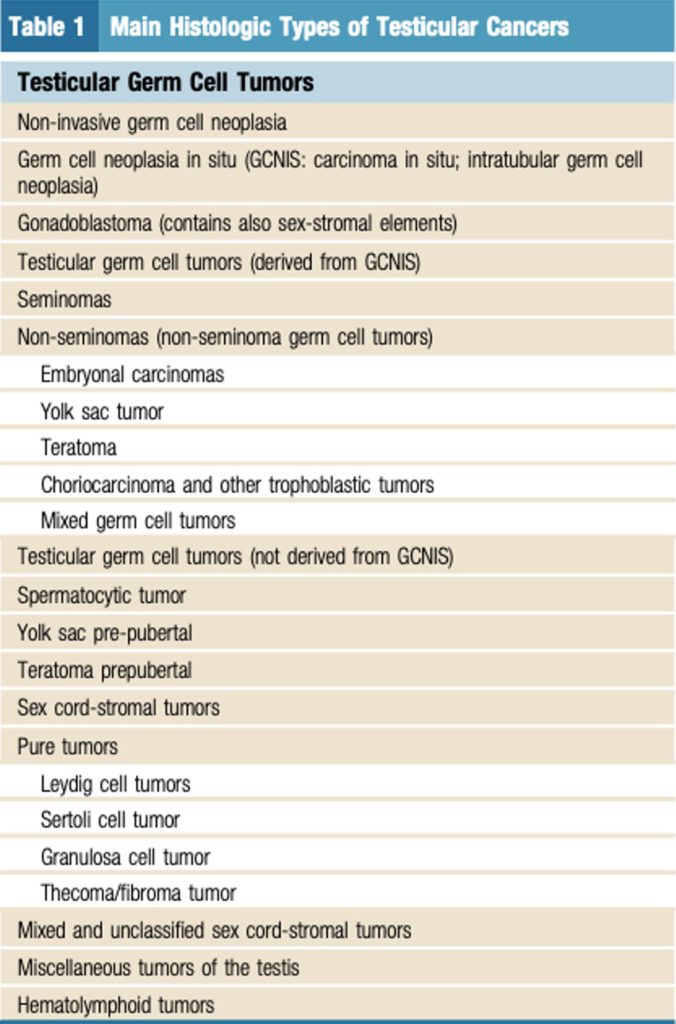
Table 1 Main Histologic Types of Testicular Cancers. Obtained from (Leão et al., 2019)
What does the prognosis for testicular cancers look like?
TGCTs have high cure rates and have an excellent prognosis in young men. However, as stated above, the current biomarkers are neither sensitive nor specific for the prognosis of testicular cancer. Hence, modern research investigating reliable circulating biomarkers could aid in early diagnosis and risk stratifying patients in terms of prognosis, relapse rates, surgical interventions and tailoring care to individualised patients. They could also reduce the need for unnecessary cross-sectional computed tomography imaging, minimise the radiation burden, and assist in follow-up choices (active surveillance vs adjuvant chemotherapy).
How is testicular cancer currently managed you ask?
Here lies the dilemma as current management and high prognosis amongst testicular cancer translate to little research into new reliable biomarkers which could be used for testicular cancer.
The management and treatment of testicular cancer are based on the TNM classification, and traditional serum tumour biomarkers (AFP released by yolk sac cells, LDH marker of bulk disease, hCG produced by trophoblasts). Table 2 shows each of the tumour marker characteristics from (Montgomery et al., 2011)
Only 60% of TGCTs actually express these elevated tumour markers at initial diagnosis, with 90% of NSGCT’s presenting with a rise in the markers but only 30% of seminomas developing a high hCG during the course of testicular cancer.
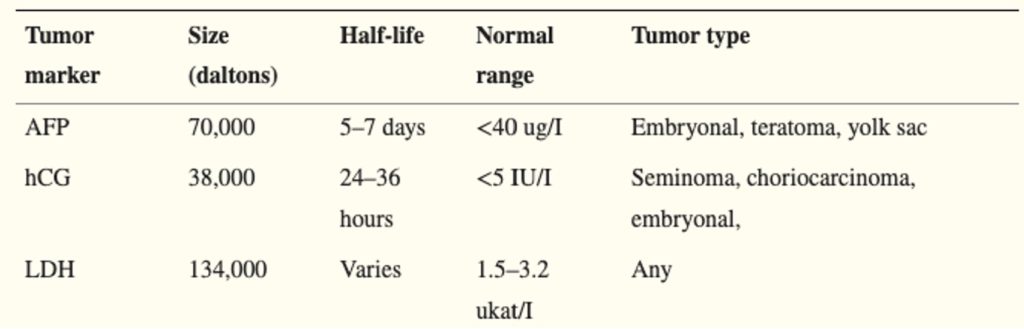
Table 2 Characteristics of Testicular Cancer Tumour Markers. Obtained from (Montgomery et al., 2011)
As stated previously, these markers are also non-specific for the diagnosis, follow-up and monitoring of testicular cancer. The elevation of these serum markers can occur due to numerous conditions , resulting in false positives, including:
AFP: HCC, viral hepatitis, cirrhosis, pancreatic cancers, anti-epileptic drugs, etc..
hCG: breast cancer, hypogonadism, bladder and kidney cancers,
LDH: PE, AMI, skeletal muscle disease etc
NSGCTs Management
Clinical Stage I (CS I) testicular cancer is defined as a localised disease, with normal serum markers. Orchiectomy is preferred for all CS I NSGCT, with 30% of patients managed with only active surveillance post-orchiectomy relapsing after the first year. Hence, post orchiectomy, patients need to be risk-stratified according to prognostic biomarkers in order to identify if adjuvant treatment is required. The majority of post-orchiectomy procedures are active surveillance, yet other adjuvant treatment options such as chemotherapy, or retroperitoneal lymph node dissection (RPLND) are considered if the patient is at high risk due to lymphovascular invasion (LVI) or embryonal carcinomas.
The problem presents itself when deciding to offer all patients CS I disease adjuvant treatment, thereby exposing them to long-term consequences of both chemotherapy and RPLND including infertility, loss of ejaculation, and infection. Even only 50% of high-risk patients, those with LVI or embryonal carcinoma, will actually progress to advanced disease, meaning risk stratification for both high and low-risk CS I patients will improve the allocation of adjuvant treatment and prevent unnecessary harm to the patients.
Seminoma Testicular Cancer Management
CS I seminomas have a high cure rate post-orchiectomy alone, with only 16.8% of all patients managed with active surveillance relapsing. Despite this, identifying high-risk patients post-orchiectomy is still not met.
New and Existing Research for alternative biomarkers for testicular cancer
Liquid biomarkers
Research is moving toward using liquid biomarkers and liquid biopsy rather than using tissue markers due to invasiveness, riskiness and often impossibility of obtaining a tumour sample. In contrast, liquid biopsies are non-invasive, are a better reflection of tumour heterogeneity than tissue-based markers, and can be used to monitor disease continuously at successive time points. There are a number of liquid biomarkers discussed in Leão et al, including placental alkaline phosphate, TRA-1-60, Neuro-specific Enolase, Lectin-reactive AFP, and N-glycans.
All of these liquid biomarkers are currently still being evaluated for their use in testicular cancer and sensitivity rates. Some are more promising including N-glycans, for the diagnosis, follow-up and evaluation of residual disease post-chemotherapy. However, further research is still being performed so I would highly encourage to watch this space.
Circulating Tumour DNA (ctDNA), Circulating Free DNA (cfDNA), and Circulating Tumour Cells (CTCs)
ctDNA, cfDNA and CTCs all present a method for non-invasive cancer biomarkers, that indicate tumour presence. ctDNA is readily available as it escapes into the circulation from the primary tumour, necrosis, apoptosis or being actively released from tumour cells. ctDNA is a real-time biomarker for diagnosis and monitoring and can be frequently collected at continuous time points to characterise disease over time. It is known that tissue sampling may not be representative of all histological features of a tumour, due to tumour heterogeneity, which is all rendered moot using ctDNA analysis.
However, challenges still remain. ctDNA is rapidly cleared from circulation due to surgery, and systemic therapy and often is present in small amounts in plasma, serum and other body fluids. Hence, highly sensitive techniques are required to obtain ctDNA and multi-marker analysis is required.
CTCs are derived from clones from the primary tumour and provide a live feed of metastasis and disease status. A recent study confirmed that CTCs were found in 18% of TGCTs, 41% of metastasis and 100% of chemo-refractory disease. CTCs are therefore promising but have not been studied extensively in testicular cancer. There may also be limited benefit in patients with minimal tumour burden.
Serum Micro RNA
This is perhaps the most exciting discovery if the field of testicular cancer and has a high likelihood of becoming the next biomarker available in the clinical setting. miRNA are promising as prognostic markers in testicular cancer as they are associated with lesion burden and chemotherapy response in advanced disease. Below, Table 2 is a summary of the role of serum miRNAs in testicular cancer disease obtained from Leão et al.
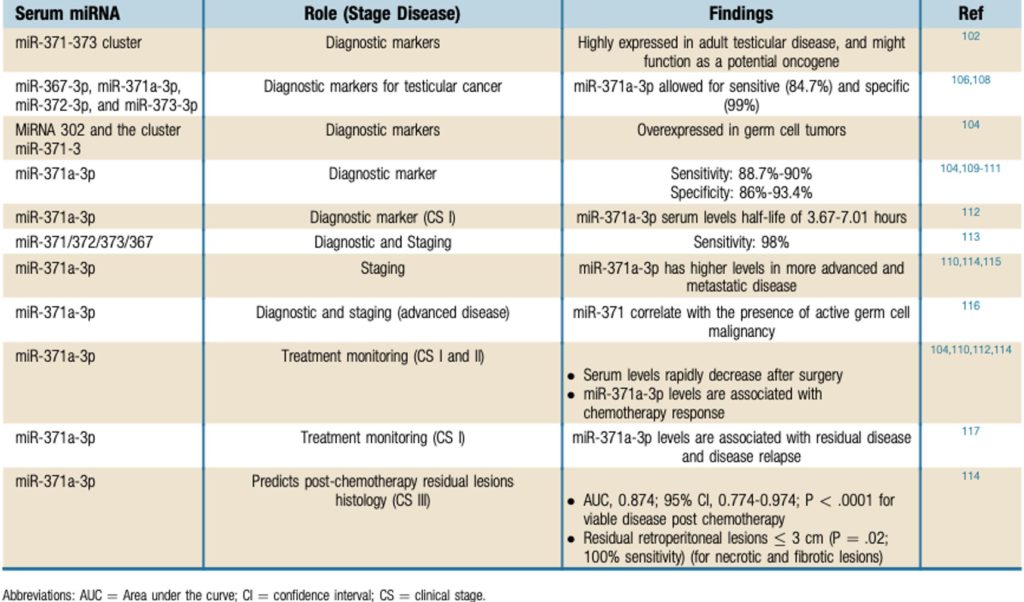
Table 3 The Role of Serum miRNAs in Testicular Cancer Disease. Obtained from (Leão et al., 2019)
Conclusions
The current serum testicular tumour markers of hCG, AFP, and LDH have had significant impacts on the diagnosis and treatment of TGCTs and are traditionally taught to us. But, as we have seen from this article, they lack sensitivity and specificity for testicular cancer diagnosis, and lack prognostic value for treatment post-orchiectomies. New research has shown other markers including ctDNA, miRNA and biomarkers could be used in the diagnosis and monitoring of testicular cancers. There are obvious challenges to this such as the limited research performed, small sample sizes and lack of testicular cancer-specific research. There are also discrepancies in the research data regarding these new markers that need to be studied extensively before these markers can be used for anything other than research purposes. Nevertheless, stay tuned and watch the exciting field of personalised medicine and serum markers as a non-invasive means of characterising cancers.
References
Leão, R., Ahmad, A. E., & Hamilton, R. J. (2019). Testicular cancer biomarkers: A role for precision medicine in testicular cancer. Clinical Genitourinary Cancer, 17(1). https://doi.org/10.1016/j.clgc.2018.10.007
Montgomery, J., Weizer, A., Filson, C., Milose, J., & Khaled Hafez. (2011). Role of biochemical markers in testicular cancer: Diagnosis, staging, and surveillance. Open Access Journal of Urology, 1. https://doi.org/10.2147/oaju.s15063



