Research Snippet #4: CAR T-Cell Cancer Therapy
Welcome to another CSM research review!
Recently there has been a lot of excitement around the use of CAR T-cells in cancer therapy, particularly after they were approved for their first use in Australia in early 2020. Recently, on the 26th of November 2020, Samadani et al. published a review paper assessing the success of CAR T-cells in cancer therapy, which gives us the perfect opportunity to re-visit how CAR T-cells have been faring as an immunotherapy agent in cancer treatment.[1]
First, let us briefly review what a CAR T-cell is. The term “CAR T-cell” is short for “Chimeric Antigen Receptor T-cell.” The term ‘chimeric’ refers to the fact that the receptor is formed from two separate components, just as a mythic chimera is formed from the parts of two animals put together. The chimeric antigen receptor, on a microscopic scale, combines the antigen-binding region of an antibody with the signalling region of a T cell receptor (TCR) complex to form a single receptor. This is formed by determining an antigen that is specific to the tumour. The next step is taking out some of the patient’s T cells and introducing a gene based on the antigen-binding region of an antibody which is specific to an antigen found within the patient’s tumour. This receptor will, like a TCR, be found on the surface of a T cell, which can be either CD4+ (‘helper’ T cells) or CD8+ (‘killer’ T cells). This creates a set of T cells that are specific to the tumour cells, which are then re-introduced to the patient as immunotherapy. The benefit of having a CAR rather than the usual TCR is that the CAR target tumour cells that have stopped expressing the MHC (major histocompatibility complex), just as an antibody is able to do. A TCR, on the other hand, can only bind to tumour antigens expressed alongside MHC.
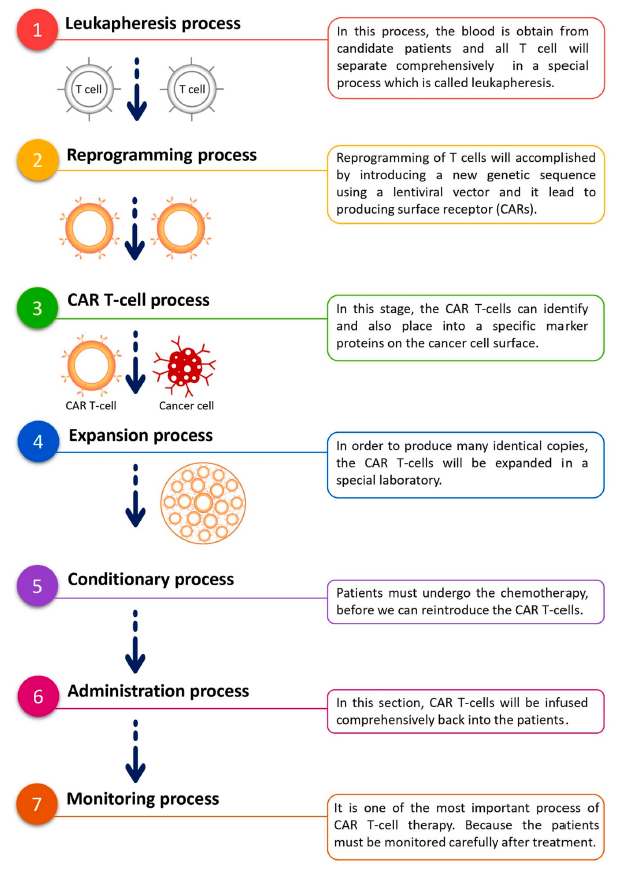
Figure 1: A diagram showing the process of CAR T-cell therapy from Samadani et al..
Samadani et al. noted several kinds of cancer in which CAR T-cell therapies are showing promise. Here are some notable examples:
- Diffuse large B cell lymphoma (DLBCL) and some types of leukaemia, by targeting the CD19 antigen that is expressed on B cells. The use of CD20, another B cell protein, to target a variety of B cell lymphomas, is also promising.
- Acute lymphoblastic leukaemia, also by targeting CD19.
- Hodgkin and non-Hodgkin lymphoma, by targeting CD30, which is a type of TNF (tumour necrosis factor) receptor that is only expressed by activated lymphocytes, haematopoietic stem cells and progenitor cells.
- Acute myeloid leukaemia, by targeting the CD33 antigen expressed on B cells, activated T cells and natural killer cells.
In the above examples, the CAR T-cells are being used to direct the T cell-mediated immune system against abnormal leukocytes. We should note that of the above, the treatments targeting CD19 and CD20 have so far been used with success in humans, whereas the others have only been tried on mouse models thus far. It is also interesting to note that the above examples are targeting haematological malignancies, rather than solid tumours. Solid tumours are harder to target with CAR T-cells, since they are more concentrated with abnormal angiogenesis limiting the circulation of CAR T-cells to tumour cells. They also exhibit much wider variation of antigens between tumour cells within the same tumour, meaning that finding an antigen receptor that can target all the cells is difficult. The physical barriers surrounding solid tumours also prevent penetration by CAR T-cells
Samadani did, however, note ways in which CAR T-cells could be used to inhibit tumour angiogenesis and growth. One example is by targeting VEGFR2 (Vascular Endothelial Growth Factor Receptor 2). This works by preventing tumour cells from forming their own blood supply, thus reducing tumour growth. Samadani noted that this is more useful for slowing tumour progression than for inducing remission. So far, this has only been done in mouse models.
As well as their ineffectiveness against solid tumours, the use of CAR T-cells is also limited by the toxicity they can cause. These include Cytokine Release Syndrome (CRS), Macrophage Activation Syndrome (MAS), neurotoxicity, and Tumour Lysis Syndrome (TLS). According to Samadani, another problem arises when the CAR T-cells target antigens that are expressed in low levels on the patient’s normal cells. They noted that this last complication was observed during a clinical trial for CAR T-cells treating renal carcinoma, where the antigen that was being targeted in the renal cells was also expressed in low levels in liver cells, causing liver toxicity.
Samadani also noted several technical difficulties with producing CAR T-cells. Another problem is that treatments which target B cells cause significant B cell aplasia and thus immunosuppression. Rather than going into detail here, I will include a table that the authors compiled to summaries the current advantages and disadvantages of CAR T-cell therapy:
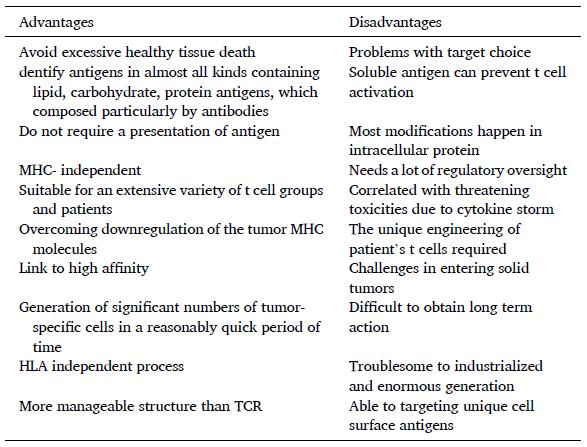
All in all, we see that CAR T-cell therapy is very promising for use in haematological malignancies, particularly leukaemia and lymphoma. However, most of these treatments are still in the animal trial or clinical trial phase, and there are still a lot of barriers preventing them from being used routinely for a wide range of cancers.
If you would like to find out more, here is a link to Samadani et al’s paper: https://pubmed.ncbi.nlm.nih.gov/33249047/
Resources:
[1] Ali Akbar Samadani International Immunopharmacology, https://doi.org/10.1016/j.intimp.2020.107201



