Research Snippet 7: Breast Cancer Spotlight
Epidemiology and Risk Factors of Breast Cancer
Breast cancer arises from the lining cells of the glandular tissue within the breast. Breast cancer is the second most commonly diagnosed cancer among females in Australia, and second highest cause of cancer mortality in the same demographic (behind lung cancer) [1]. For obvious reasons, female gender has the strongest association with breast cancer, however, it must not be forgotten that breast cancer can also affect men (0.5%-1% of total cases) [2]. Breast Cancer can have an association with family history, although the majority arise in individuals without a family predisposition.
The marked incidence of most cancers with age, means that it’s now standard for all women over the age of 50 to be screened for breast cancer with mammograms. The exception being a minority of individuals with “high penetrance genetic mutations” (e.g. BRCA1, BRCA2) which justify an earlier and more diligent screening schedule, and possibly even preventative surgical removal of breast tissue.
On top of age and genetics, lifestyle factors contributing to the aetiology of breast cancer are beginning to be unravelled. It’s been public knowledge that smoking and excessive alcohol use contribute to a constellation of cancers throughout the body, including breast cancer. But only in recent years has evidence linked obesity and metabolic syndrome as a leading driver of breast cancer. Renehan et al’s study published in the Lancet, concluded that every “5-unit increase in BMI was correlated with a 12% increase in the risk of breast cancer” [3]. The mechanistic underpinnings of the correlation is still an active area of research. Some hypotheses implicate “low level chronic inflammation” that is often seen in fat tissue surrounding visceral organs and breast tissue, others refer to the role of newly-named “adipokines” (hormones released from adipose/fat tissue) which act locally to disrupt metabolic signalling in adjacent breast tissue, and others yet suggest a role of fat cells releasing estrogen (an established iatrogenic risk factor for breast cancer, pertinent to individuals placed on “estrogen hormonal therapy”) [4, 5].
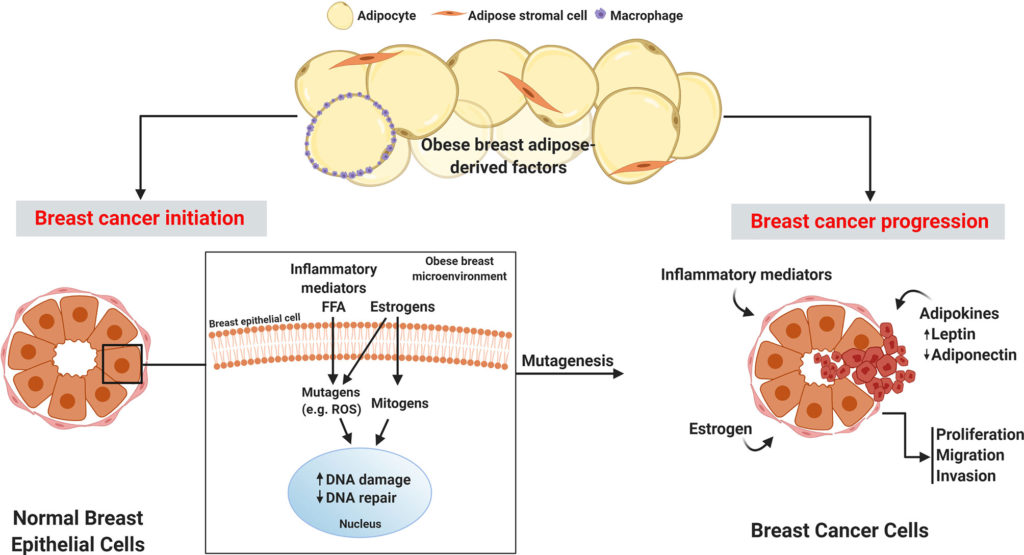
Figure 1: Obese breast adipose-derived factors contribute to the initiation and progression of breast cancer.
Retrieved 2 August 2021 from [5]: Bhardwaj P, Brown K. Obese Adipose Tissue as a Driver of Breast Cancer Growth and Development: Update and Emerging Evidence. Frontiers in Oncology. 2021;11.
Classification of Breast Cancer
It is important to remember that breast cancers are heterogeneous in nature and different breast cancer subtypes have variable responses to treatment and clinical behaviour. The classification of breast cancers is important for oncological diagnosis, prognosis, management and decision making.
Traditional Classifications
Histological classification
Traditional classifications of breast cancers include the histologic subtyping into infiltrating ductal carcinomas, no special type (IDC-NST) which represent 70-80% of all invasive breast cancers [6]. This is followed by invasive lobular carcinomas (ILC) which account for 10% of breast cancers [6]. The remaining percentage includes rare histological subtypes including metaplastic, apocrine, cribriform, micropapillary, papillary, medullary, tubular and mucinous carcinomas. Histological classification is based on: cell type, extracellular secretions, architecture, and immunohistochemical (IHC) profile of cancer [6].
However, classifying breast cancers into the same histological type does not indicate they have the same biological and clinical behaviour. Rather, this classification is a broader categorisation system rather than a detailed one that does not reflect the true heterogeneity within breast cancers [6].
Immunophenotype
Three receptors are routinely assessed in breast cancers: estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor-2 (HER2). Classifying breast cancers according to their receptor positivity aids in prognosis and are predictive markers for successful targeted and hormonal therapy [6].
ER and PR are sex steroid nuclear receptors and are assessed via IHC for nuclear expression. When activated they both promote the growth of the neoplastic breast epithelium [6, 7]. The majority of breast cancers that are ER+ are also PR+, and usually represent low grade, non-aggressive cancers. ER+/PR+ breast cancers represent 75% of breast cancers, whereas 15% of breast cancers also are HER2+ [6]. HER2 is assessed using IHC and DNA in situ hybridization procedures and HER2+ positive breast cancers are usually more aggressive with a poorer prognosis than ER+/PR+ breast cancers. Finally, ER-/PR-/HER2- are triple-negative breast cancers (TNBC) that represent 10-15% of all breast cancers [6]. These receptor categorizations were helpful in the development of the molecular classification of breast cancers [7].
Molecular Classification/Intrinsic Subtype
With advancing technology, the extensivity of breast cancer heterogeneity has become better understood, especially at the molecular level which has led to a different breast cancer classification system [7]. Perou and Sorlie were responsible for proposing the molecular classification of breast cancers, which has a higher ability to determine the prognostic and predictive features of breast cancers in comparison to traditional methods [6]. It came to fruition with the advent of genomic/expression profiling, high-throughput technologies and micro-array based applications, which enabled the classification of intrinsic tumour biology rather than just morphology or receptor status alone [6,7].
There are five molecular subgroups of breast cancers:
Luminal A and Luminal B
These breast cancers are ER+ and have gene expression profiles similar to normal luminal breast cells. The genes found are those activating ER and leading to proliferation [7]. Luminal A is the most common, representing almost 50% of all molecular subgroups, and in comparison to Luminal B, is low grade and less aggressive [6].
HER2- Enriched
This reflects breast cancers over-expressing/amplifying HER2 [7]. It represents 15% of all molecular subtypes and represents the cancers overexpressing HER2 signalling genes [6]. They tend to be high grade and aggressive tumours.
Basal
This reflects TNBC that also express genes found in normal basal (myoepithelial) breast cells and have a high amount of proliferation-related genes [6]. They have a high proliferation index, with the lowest prognosis out of all molecular subtypes, especially recurrence after 5 years of diagnosis [6, 7].
Normal breast-like
These tumours have the gene expressions found in normal breast epithelium. There has been controversy surrounding this group, especially those who state that it was normal breast tissue that was contaminated [6, 7].
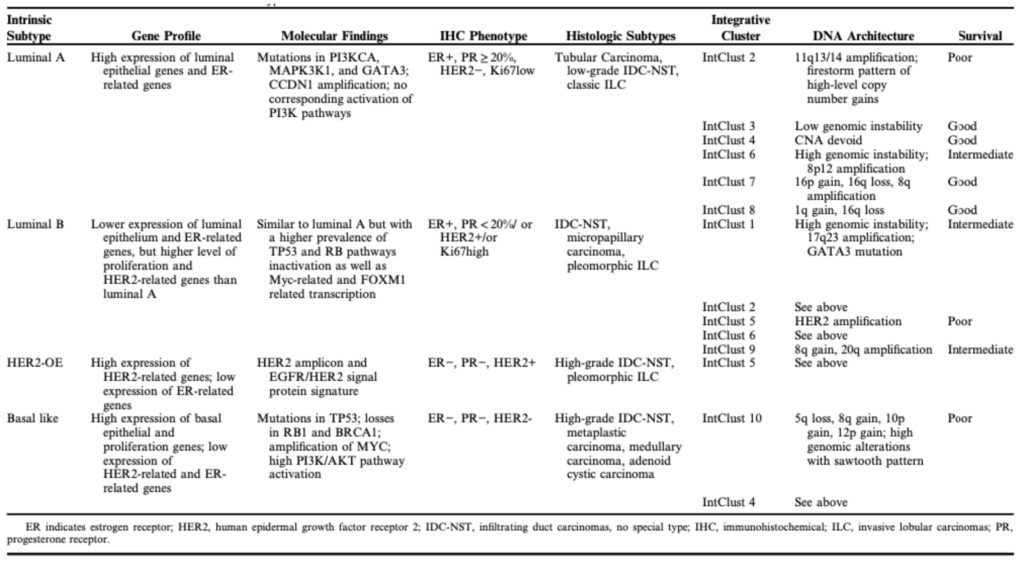
Table 1 Overview of Molecular Subtypes of Breast Cancer.
Retrieved 2 August 2021 from [6] Tsang J, Tse GM. Molecular classification of breast cancer. Advances in anatomic pathology
Treatment and Prognosis of Breast Cancer
Breast cancers like many other solid tumour cancers are treated with an arsenal of pharmacological and surgical approaches. Broadly speaking, treatment options can be subcategorized into local treatments (those that treat the tumour in isolation) and systemic treatments (those which treat cancers that have often spread locally or metastasized). With a greater understanding of the molecular and genetic markers that separate different types of breast cancer, treatment options have become increasingly diverse and personalised. Here we will explore the various indications for certain treatment options and shine a light on recent developments in personalised therapeutic agents which target cell cycle pathways, arresting breast cancers in their tracks – CDK4/6 inhibitors.
Surgical techniques have often been the mainstay of the treatment of localised breast cancers. Here, either breast-conserving surgery is often performed with the aim of removing as much if not all of the breast cancer whilst leaving as much normal breast tissue as possible. Should this not be indicated (generally if the patient has genetic risk factors such as BRCA or ATM mutations), a total mastectomy may also be indicated [8]. Radiation therapy is often used in conjunction with surgical treatment to minimise the possibility of recurrence. Depending on the extent of cancer, regional lymph nodes are also assessed and excised/biopsied during surgery either as an explorative or curative measure. Surgical approaches are however generally not conducted in isolation without neoadjuvant or adjuvant systemic therapies. The major side effect of surgical approaches is generally lymphoedema – a major impediment to the quality of life of many breast surgery patients [9].
There are also a variety of systemic chemotherapeutic agents that can be used for patients. Oftentimes there are either neoadjuvant therapies, meaning they are delivered before the main treatment modality to minimise the size of cancer or adjuvant therapies, which are given in conjunction with or after the main treatment phase. Irrespective of timing, the types of therapies are similar between the two. Broadly speaking, drug treatment can be categorised into chemotherapy, hormone therapy, immunotherapy and targeted drug therapy.
Hormone therapy such as tamoxifen is often reserved for cancers where there is a known endocrine or hormonal driver of disease. These drugs can either act at the receptor level, acting as a brake, preventing the endogenous hormone from interacting with the receptor and driving cancer growth, or at the hormone level, reducing endogenous levels of the said hormone within the body. For example, tamoxifen is a selective estrogen receptor modulator that blocks estrogen receptors and thus breast cancer division. Immunotherapies function to ramp up a patient’s immune system to recognise and attack cancer cells more effectively.
CDK4/6 inhibitors
CDK4/6 inhibitors have been touted as the new modality for treating breast cancer.
Whilst CDK4/6 inhibitors like abemaciclib have been shown to act at the cell cycle level preventing tumour cell growth, new research has illustrated how powerful these therapies can be at modifying the tumour landscape [10]. In animal models, CDK4/6i has been shown to upregulate antitumour immunity. More specifically these antitumour effects have been shown to be mediated through a two-pronged approach. CD4/6 inhibitors not only act on tumour cells themselves by increasing antigen processing and presentation leading to increased immunogenicity and susceptibility to cytotoxic T cells, it also suppresses tumour cell proliferation by upregulating regulatory T cells [10]. In combination, these two effects work to ramp up the ability of the immune system to detect and eliminate aberrant cancer cells. In doing so, these therapies hit at the core of what drives cancer cells and their escape from our immune system.
Concluding Remarks
Breast cancer is an aggressive solid tumour affecting many families every single year. With new advances in diagnosis and staging, coupled with advances in basic science research to understand the biology of cancer, clinicians and scientists alike are getting closer to creating safe and effective treatment modalities to deal with this cancer.
References
[1]: Breast cancer in Australia statistics | Cancer Australia [Internet]. Canceraustralia.gov.au. 2021 [cited 2 August 2021]. Available from: https://www.canceraustralia.gov.au/affected-cancer/cancer-types/breast-cancer/breast-cancer-australia-statistics
[2]: Breast cancer [Internet]. Who.int. 2021 [cited 2 August 2021]. Available from: https://www.who.int/news-room/fact-sheets/detail/breast-cancer
[3]: Renehan A, Tyson M, Egger M, Heller R, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. The Lancet. 2008;371(9612):569-578.
[4]: Obesity and Cancer Fact Sheet [Internet]. National Cancer Institute.
2021 [cited 2 August 2021]. Available from: https://www.cancer.gov/about-cancer/causes-prevention/risk/obesity/obesity-fact-sheet
[5]: Bhardwaj P, Brown K. Obese Adipose Tissue as a Driver of Breast Cancer Growth and Development: Update and Emerging Evidence. Frontiers in Oncology. 2021;11.
[6]: Tsang J, Tse GM. Molecular classification of breast cancer. Advances in anatomic pathology. 2020 Jan 6;27(1):27-35.
[7]: Eliyatkin N, Yalcin E, Zengel B, Aktaş S, Vardar E. Molecular classification of breast carcinoma: From traditional, old-fashioned way to a new age, and a new way. Journal of Breast Health. 2015;11(2):59–66.
[8]: Cancer.org. 2021. Breast Cancer Treatment | Treatment Options for Breast Cancer. [online] Available at: <https://www.cancer.org/cancer/breast-cancer/treatment.html> [Accessed 4 August 2021].
[9]: Ayre, K. and Parker, C., 2019. Lymphedema after treatment of breast cancer: a comprehensive review. Journal of Unexplored Medical Data, 2019.
[10]: Goel, S., DeCristo, M., Watt, A. et al. CDK4/6 inhibition triggers anti-tumour immunity. Nature 548, 471–475 (2017).



